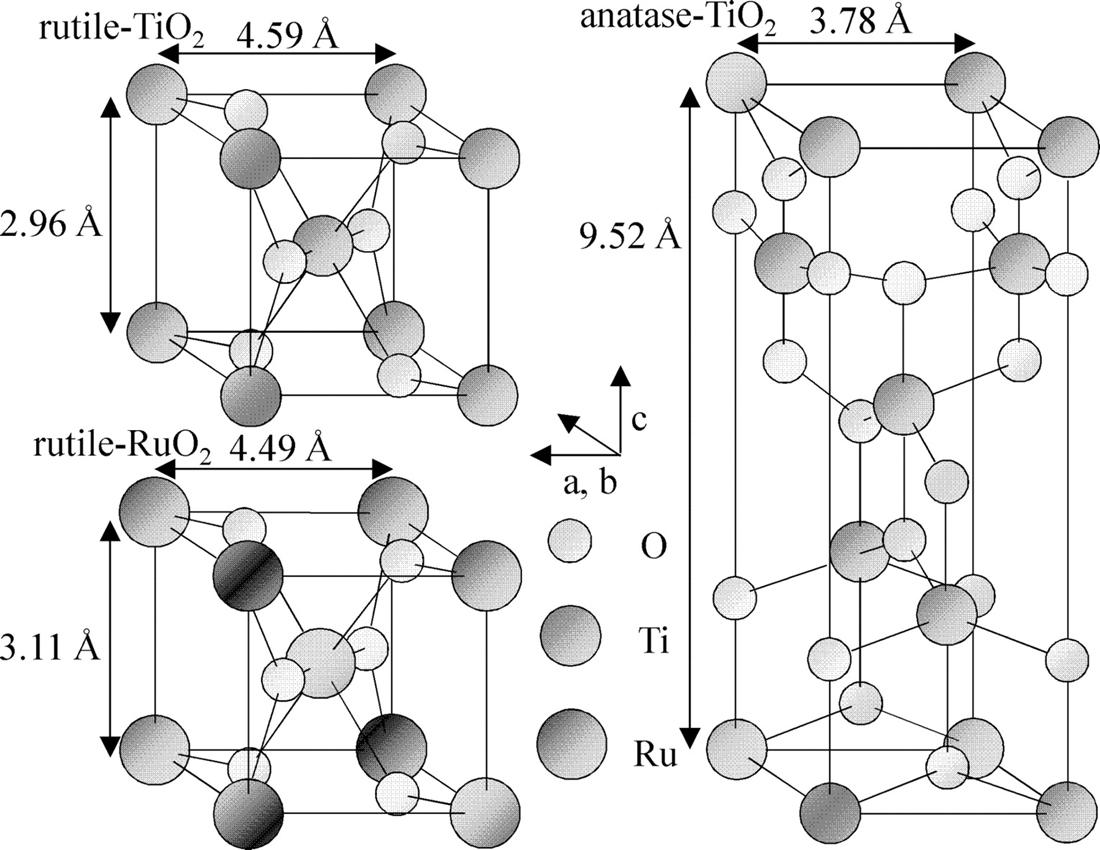
What is the difference between rutile and anatase titanium dioxide?
Titanium dioxide has two forms: Rutile and Anatase
Rutile and anatase are both used in the white pigmentation of paints, paper, and ceramics. Rutile and anatase provide extra luster to other gems and minerals since they are capable of asterism.
What is the difference between rutile and anatase titanium dioxide?
Rutile is deep red while anatase is yellow to blue.
Rutile has a high absorbance property than anatase.
 |
 |
Rutile titanium dioxide
Rutile titanium dioxide is the most common form. It is preferably used in interference applications because of its high refractive index. It is also hard and has chemical resistant properties.
Anatase titanium dioxide
Anatase titanium dioxide is a type of polymorph which becomes a rutile when it is exposed at about 915 degrees centigrade. Its color is brown to black or yellow to blue. Anatase is the rarest form of titanium dioxide, but it has almost the same properties as rutile with regards to its hardness, density, and luster.