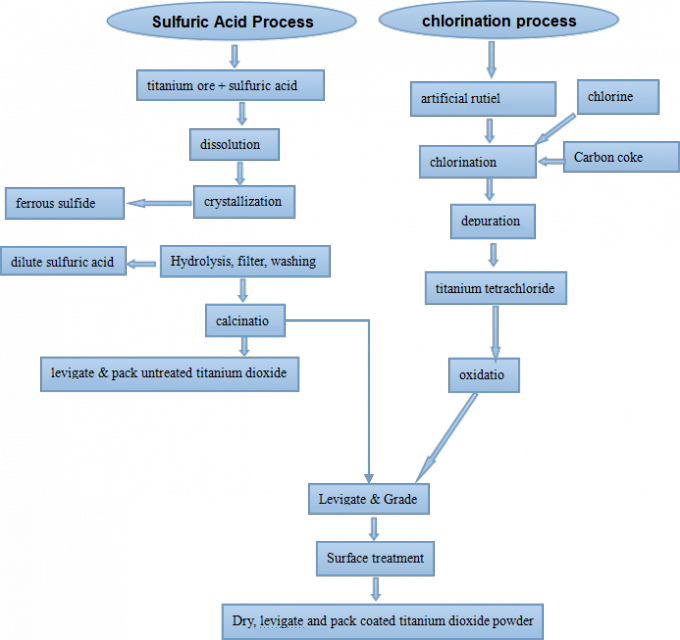
How to manufacture titanium dioxide by chloride process
How is pure titanium dioxide manufactured?
There are two main production methods to converte titanium containing ores into pure titanium oxide: The sulfate process and the chloride process.
Chloride process
Also called: Chlorination process; Chlorin-ation route; Chloridize style
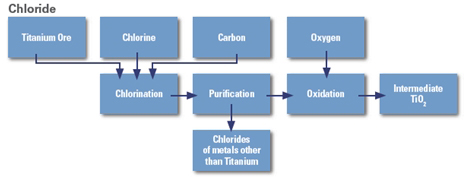
1. The chloride process requires purer ore or rutile which is a much rarer. The raw material must contain at least 70% rutile. Titanium dioxide is reduced with carbon and then oxidised again with chlorine.
TiO2 + C → Ti + CO2
Ti + 2Cl2 → TiCl4
2. Liquid TiCl4 is distilled off and converted back into TiO2 in a pure oxygen flame or in plasma at temperatures of 1200–1700 °C. The majority of chlorine is recovered.
TiCl4 + O2 → TiO2 + 2Cl2
This process also uses a large amount of hazardous chemicals and substantial quantities of energy. Apart from solid or liquid waste of unreacted minerals or different chlorine compounds, the chloride process can produce gaseous particulates, chlorine and sulphur dioxide emissions. The chloride process has been favoured on financial and environmental grounds since the early 1990s.
How to manufacture titanium dioxide by chloride process
The chloride process uses titanium-containing raw materials, reacts with chlorine gas such as chlorinated high-titanium slag or artificial rutile, or natural rutile to form titanium tetrachloride, which is purified by rectification and then subjected to gas phase oxidation; after rapid cooling, After gas-solid separation, TiO2 is obtained.
The TiO2 is adsorbed by removing a certain amount of chlorine by heating or steam treatment. The process is simple, but chlorination at 1000° C or higher, there are many chemical engineering problems such as chlorine, oxychloride, titanium tetrachloride, high corrosion needs to be solved, plus the special materials used, compared to the cost of sulfuric acid high.
The production of chloride process is continuous production. The flexibility of the operation of the production equipment is not large. The opening and stopping of the production and the production load are not easy to adjust, but the continuous process production, the process is simple, the process control point is small, and the product quality is easy to achieve optimal control.
In addition, there is no sintering formed by the rotary kiln calcination process, and the TiO2 primary particles are easily depolymerized, so it is customary to think that the quality of the chlorinated titanium dioxide product is superior.
The advantage of the chloride process
The advantage of the chloride process is that the process is short, the production capacity is easy to expand, the degree of continuous automation is high, the energy consumption is relatively low, and the "three wastes" are few, and high quality products can be obtained.
The shortcomings of the chloride process
The shortcomings of the chloride process are large investment, complicated equipment structure, high material requirements, high temperature resistance, corrosion resistance, difficult to repair the device, and difficult research and development.
