How to manufacture titanium dioxide by sulfate process
How is pure titanium dioxide manufactured?
Once the titanium containing ores have been mined, they need to be converted into pure titanium oxide.
The two main production methods are the sulfate process and the chloride process.
Sulfate Process
Other names: Soleplate process, sulfuric acid method, sulphate process; sulferic acid;
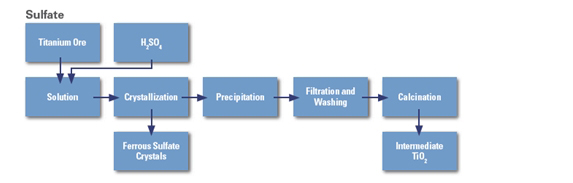
How to manufacture titanium dioxide by sulfate process
The iodine powder is reacted with concentrated sulfuric acid to produce titanyl sulfate, which is hydrolyzed to form metatitanic acid, and then calcined and pulverized to obtain a titanium white powder product. This method can produce anatase and rutile titanium dioxide.
In the sulfate process, ilmenite (FeTiO3), a common iron/titanium oxide material, is used. It is treated with concentrated sulfuric acid (H2SO4) and the titanium oxygen sulfate (TiOSO4) is selectively extracted and converted into titanium dioxide.
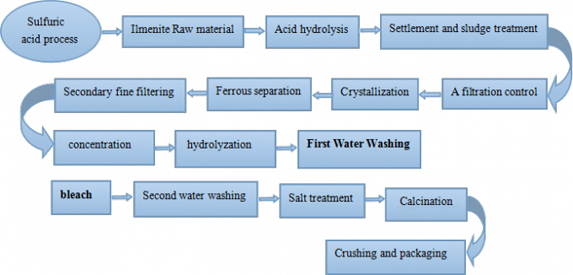
1. Ilmenite is treated (digested) with a 60% excess of concentrated sulfuric acid at a temperature around 100 °C. The following reaction takes place:
FeTiO3 + 2H2SO4 → FeSO4 + TiOSO4 + 2H2O
2. In the next stage, the waste product iron(II)sulfate is removed. As FeSO4 is not very soluble at low temperatures, the solution is cooled to around 15 °C and FeSO4 crystallises out. It can then be removed by filtration.
The remaining aqueous digestion products are heated to around 110 °C in order to hydrolyse the titanium oxygen sulphate.
TiOSO4 + (n+1)H2O → TiO2•nH2O + H2SO4
The hydrolysis stage of the process produces sulfuric acid waste and a precipitate gel containing hydrated titanium dioxide.
3. In the last stage, the hydrated titanium dioxide is heated in large rotary kilns to drive off the water and produce crystals of anatase or rutile (2 forms of titanium dioxide).
TiO2•nH2O → TiO2 + nH2O
Water is removed at temperatures between 200–300°C. Seed crystals are added to start the crystallisation process. Depending on the final heating temperature (800−850°C or 900−930°C), either anatase or rutile is formed, respectively.
The sulfate process requires the use of very large quantities of sulfuric acid and produces copious amounts of acidic waste. This acidic waste could cause significant damage to the environment, if not disposed of responsibly.
The advantage of the sulfate process
The advantage of the sulfate process is that the ilmenite and sulfuric acid which are low in price are available as raw materials, the technology is mature, the equipment is simple, and the anti-corrosion material is easy to solve.
The shortcoming of the sulfate process
The shortcoming of the sulfate process is that the process is long and can only be based on intermittent operation, wet operation, high consumption of sulfuric acid and water, and many wastes and by-products, which are harmful to the environment.
